| The Royal Swedish Academy of
Sciences has awarded
The 1998 Nobel Prize in Chemistry in the area of quantum chemistry to
Walter Kohn,
University of California at Santa Barbara, USA and
John A. Pople,
Northwestern University, Evanston, Illinois, USA (British citizen).
The Laureates have each made pioneering
contributions in developing methods that can be used for theoretical studies of the
properties of molecules and the chemical processes in which they are involved.
Citation:
"to Walter Kohn for his development of the density-functional theory and to John
Pople for his development of computational methods in quantum chemistry."
Development of computational
methods in chemistry awarded
Researchers have long sought methods for
understanding how bonds between the atoms in molecules function. With such methods it
would be possible to calculate the properties of molecules and the interplay between them.
The growth of quantum mechanics in physics at the beginning of the 1900s opened new
possibilities, but applications within chemistry were long in coming. It was not
practically possible to handle the complicated mathematical relations of quantum mechanics
for such complex systems as molecules.
One of the founders of quantum physics, Dirac,
expressed the problem in 1929 as follows: "The fundamental laws necessary for the
mathematical treatment of large parts of physics and the whole of chemistry are thus fully
known, and the difficulty lies only in the fact that application of these laws leads to
equations that are too complex to be solved.
Things began to move at the beginning of the 1960s
when computers came into use for solving these equations and quantum chemistry (the
application of quantum mechanics to chemical problems) emerged as a new branch of
chemistry. As we approach the end of the 1990s we are seeing the result of an enormous
theoretical and computational development, and the consequences are revolutionising the
whole of chemistry. Walter Kohn and John Pople are the two most prominent
figures in this process. W.Kohn's theoretical work has formed the basis for simplifying
the mathematics in descriptions of the bonding of atoms, a prerequisite for many of
today's calculations. J. Pople developed the entire quantum-chemical methodology now used
in various branches of chemistry.
Computer-based calculations are now used generally
to supplement experimental technics. For several decades they have been developed and
refined so that it is now possible to analyse the structure and properties of matter in
detail. Conventional calculation of the properties of molecules is based on a description
of the motion of individual electrons. For this reason, such methods are mathematically
very complicated. Walter Kohn showed that it is not necessary to consider the
motion of each individual electron: it suffices to know the average number of electrons
located at any one point in space. This has led to a computationally simpler method, the density-functional
theory. The simplicity of the method makes it possible to study very large molecules.
Today, for example, calculations can be used to explain how enzymatic reactions occur. It
has taken more than thirty years for a large number of researchers to render these
calculations practicable, and the method is now one of the most widely used in quantum
chemistry
John Pople is rewarded for developing
computational methods making possible the theoretical study of molecules, their properties
and how they act together in chemical reactions. These methods are based on the
fundamental laws of quantum mechanics as defined by, among others, the physicist E.
Schr�dinger. A computer is fed with particulars of a molecule or a chemical reaction and
the output is a description of the properties of that molecule or how a chemical reaction
may take place. The result is often used to illustrate or explain the results of different
kinds of experiment. Pople made his computational technics easily accessible to
researchers by designing the GAUSSIAN computer program. The first version was published in
1970. The program has since been developed and is now used by thousands of chemists in
universities and commercial companies the world over.
Quantum chemistry - a background
.
The laws of quantum mechanics as formulated more than 70 years ago make it theoretically
possible to understand and calculate how electrons and atomic nuclei interact to build up
matter in all its forms. The task of quantum chemistry is to exploit this knowledge to
describe the molecular system. This has proved easier said than done. It was not until the
beginning of the 1960s that development really started, when two events became decisive.
One was the development of an entirely new theory for describing the spatial distribution
of electrons, and the other was the use of the increasing potential offered by the
computer. Walter Kohn showed in 1964 that the total energy for a system described
by the laws of quantum mechanics can be theoretically calculated if the electrons' spatial
distribution (electron density) is known. The question is only how the energy
depends on the density. Kohn gave important clues based on what this dependence looked
like in an imaginary system with free electrons. It was to take several decades and
contributions from many researchers, however, before the equation for determining the
energy was sufficiently accurately mapped to permit large-scale studies of molecular
systems. This has taken place partly through the adaptation of a small number of variables
to experimental data. The method Kohn introduced came to be known as the
density-functional theory. It is now used in studies of numerous chemical problem
areas, from calculating the geometrical structure of molecules (i.e. bonding distance and
angles) to mapping chemical reactions.
During the 1960s many research groups in Europe
and the USA started feverishly to exploit the great potential of the computer. New methods
of computation were developed and refined. John Pople was a leading figure in this
field. He realised that if theoretical methods were to gain any significance within
chemistry it was necessary to know how accurate the results are in any given case. In
addition, they must be easy to use and not too demanding of resources. Through significant
improvements in the theoretical methodology at the end of the 1960s Pople designed a
computer program which at a number of decisive points was superior to others' efforts. The
prerequisites mentioned above could now be fulfilled and GAUSSIAN-70, as the program was
called, very soon became widely used. Pople continued during the 1970s and 1980s to refine
the methodology, at the same time building up a well-documented model chemistry.
Here he was able at the beginning of the 1990s to include Kohn's density-functional
theory. By these means new possibilities opened up for analysing ever-more complex
molecules.
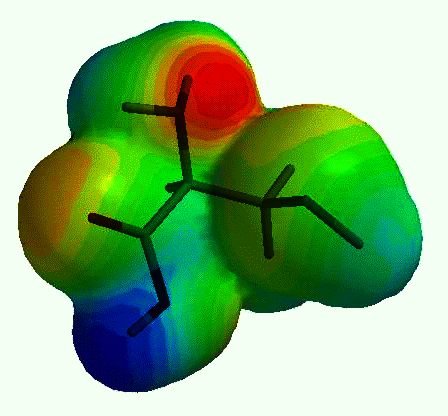
Fig. 1. Electron density in the amino acid
cystein calculated using a quantum-chemistry computer program. The picture shows the
surface where the electron density is 0.002 electrons/�3
(meaning that nearly all electrons are inside the surface). The grey scale shows the
electrostatic potential at this surface, darker portions representing negative potential.
Applications of quantum chemistry
Quantum chemistry is today used within all
branches of chemistry and molecular physics. As well as producing quantitative information
on molecules and their interactions, the theory also affords deeper understanding of
molecular processes that cannot be obtained from experiments alone. Theory and
experimentation combine today in the search for understanding of the inner structure of
matter. How is then a quantum-chemical calculation carried out?
Let us take the example of the amino acid cystein,
illustrated above. How do we produce that image? We sit in front of the computer and start
the quantum chemistry program. From the menu we select a molecule in which a carbon atom
(C) is bound to a hydrogen atom (H), an amino group (NH2),
a thiolathomethyl group CH2SH) and a carboxyl group (COOH). The computer draws a rough
picture of the molecule on the screen. We now instruct the computer to determine the
geometry of the molecule with a quantum-chemical calculation. This can take a minute or so
if we are content with a rough result, but up to a day if we desire high accuracy. The
screen picture gradually changes towards greater accuracy up to a predetermined level.
When this operation is finished we can ask the computer to calculate different properties
for the system. In the illustration above we have calculated a surface with constant
electron density. The surface is coloured according to the value of the electrostatic
potential. This can be used, for example, to predict how the molecule interacts with other
molecules and charges in its environment. Such information may be used to study how
proteins (which are built up of amino acids) interact with different substrates, e.g. in
pharmaceuticals.
Another example may be taken from the universe, in
which, apart from stars and planets, there are great quantities of interstellar matter,
often collected in vast clouds. What does this matter consist of? It can be studied from
the Earth through the radiation the molecules emit. The radiation occurs because the
molecules rotate. Hence it is possible using the frequency spectrum of the radiation to
determine the composition and appearance of the molecules. This, however, is an immensely
difficult task, particularly since these molecules cannot always be produced in the
laboratory so as to obtain material for comparative studies. Quantum chemistry, however,
does not suffer from such limitations. Calculations based on assumed structures can give
information on radio emission frequencies that can be directly compared with data
collected by the radio telescope. In this way, theory and measurement together can give
information on the molecular composition of interstellar matter.
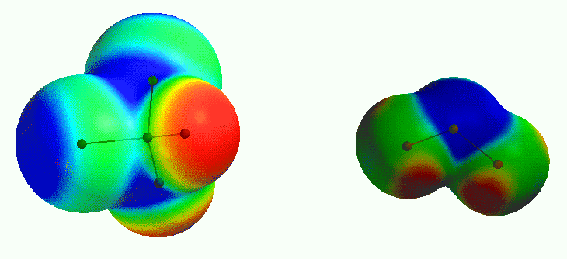
Fig 2. High up in the atmosphere, CF2Cl2 molecules (freon, left in picture) are destroyed by
ultraviolet light. Free chlorine atoms are formed, which react with O3 molecules (ozone, right in picture) and destroy them. The process can be
studied using quantum-chemical calculations.
Another example. High up in the atmosphere there is a thin layer of ozone molecules that
protect us from ultra-violet radiation from the sun. Substances that we release into the
atmosphere (e.g. freons) can lead to the destruction of the ozone layer. How does it
happen? Which chemical reactions are involved? With quantum-chemical computation we can
describe them in detail and thus understand them. This knowledge may help us to take steps
to make our atmosphere cleaner.
Quantum chemistry is used nowadays in practically
all branches of chemistry, always with the aim of increasing our knowledge of the inner
structure of matter. The scientific work of Walter Kohn and John Pople has been crucial
for the development of this new field of research.
Further reading
Additional background material on the Nobel Prize in Chemistry 1998, The Royal
Swedish Academy of Sciences, below
E. K. Wilson, Theoretical Chemistry Expands and Diversifies Across Chemical Disciplines,
Chemical & Engineering News, August 19, 1996.
J. H. Krieger, Computational Chemistry Impact, Chemical & Engineering News, May
12, 1997.
W. J. Hehre, L. Radom, P. v. R. Schleyer och J. A. Pople, Ab Initio Molecular Orbital
Theory, John Wiley & Sons, New York, 1986.
R. G. Parr and W. Yang, Density-Functional Theory of Atoms and Molecules, Oxford
Science, Oxford, 1989.
Encyclopaedia of Computational Chemistry (ed. Paul v. R. Schleyer), John Wiley
& Sons,
New York, 1998.
******
Walter Kohn was born in Vienna, Austria, in 1923. He was a professor at the
Carnegie Institute of Technology in Pittsburgh, USA between 1950 and 1960 and at the
University of California in San Diego from 1960 to 1979. He was Director of the Institute
for Theoretical Physics in Santa Barbara, where he is still active, from 1979-1984.
Professor Walter Kohn
Department of Physics
University of California at Santa Barbara
CA 93106, USA
John A. Pople was born in Burnham-on-Sea in Somerset, U.K. in 1925. British
citizen. He became Ph.D. in Mathematics at Cambridge, U.K., in 1951. In 1964 he became
Professor of Chemical Physics at Carnegie-Mellon University, Pittsburgh, USA and
subsequently Professor of Chemistry at Northwestern University, USA, in 1986, where he is
still active.
Professor John A. Pople
Northwestern University
Department of Chemistry
2145 Sheridan Road
Evanston, IL 60208, USA |


